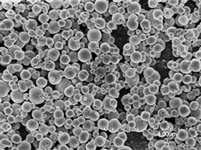
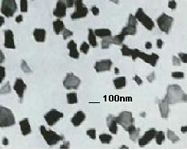
PhosphorDotsTM: Our phosphor-based lanthanide nanomaterials can be used in a variety of quantum dot-type applications,
and are particularly suitable for medical and pharmaceutical research:
-
Fluorescent nanocrystal taggant
-
Enzyme-linked immunosorbent assay (ELISA)
-
Nanoparticle labels in bioaffinity assays
-
Fluorescent/fluorogenic biology labels that can be used in multiplexing (single excitation with multiple emission)
-
Particle tracking in a live cell
-
Tracking of cells
-
In vivo/in vitro imaging and mapping
-
Biological staining of cells and tissues
-
Proteomics
-
Drug delivery diagnostics

Typical Cadmium Quantum Dot Structure vs. PhosphorDotsTM Structure
Click here to download the PhosphorDotsTM brochure.
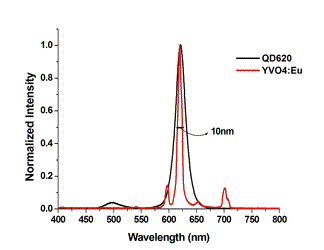
YVE1005 Emission Spectra
|
|
 Downconverting PhosphorDotsTM shown under UV excitation
Downconverting PhosphorDotsTM shown under UV excitation
New IR Upconversion PhosphorDotsTM
NIR excitation probes such as upconversion nanocrystals are preferable for both in vitro and in vivo imaging, because NIR range excitation
(750-1000nm) allows deeper light penetration with reduced light scattering, increasing image contrast.
By contrast, if UV excitation is used, biomolecules such as green-fluorescent proteins and other fluorescent organic molecules are excited
by the UV radiation and light up, interfering with image creation and capture. Excitation in NIR induces only a very weak autofluorescence
background and avoids photodegradation in biotagging applications, thus simplifying the detection of labeled target molecules and increasing the sensitivity of the method.
With high chemical stability, remarkable light penetration depth, and the absence of autofluorescence in biological specimens under
infrared excitation, upconversion nanocrystals are ideal for use as luminescent probes in biological labeling and imaging technology,
multiplexing, and bio-molecule detection.
|
| |
Old-Fashioned Quantum Dots |
Sun Innovation's Phosphor DotsTM |
| Toxicity |
Toxic without external shell protection through gradual releasing of cadmium or lead. Could be toxic for long-term applications even being insulated by a shell. |
Has no cadmium, lead, or mercury |
| Lifetime & durability |
Shell layer required to protect core from oxidization and emission quenching, increasing cost and manufacturing complexity |
No risk of oxidization since Phosphor DotsTM are an oxide, and have high emission yield without requiring complex surface treatments. Less manufacturing steps reduces number of steps in which variation, manufacturing tolerances, or defects can occur. |
| Solubility |
Solubility in water is generally bad without additional chemical modifications to render a hydrophilic surface. Most biological applications are in aqueous system. |
Prepared in aqueous solution, so solubility is not a problem. |
| Size vs. Color |
Emission color is size dependent. |
Color is not size dependent. Particle size can be tailored for different biological applications with same detection technology. |
| UV absorption |
Broad UV absorption. Tunable colors emission from 400 nm to 900 nm. |
Narrow UV absorption. |
| Excitation |
Simultaneous excitation due to broad UV excitation |
Simultaneous excitation due to same host UV absorption. |
| Synthesis |
Synthesis is complicated, such that scaling up production could be difficult. |
Simpler synthesis which can be easily scaled up to mass production. |
| Functioning |
More steps such as ligand exchange are required to add functional groups. |
No ligand exchange required; easy to add functionalization.
Available either with or without COOH functionalization. |